-
PDF
- Split View
-
Views
-
Cite
Cite
Stuart J Nelson, Kelly Zeng, John Kilbourne, Tammy Powell, Robin Moore, Normalized names for clinical drugs: RxNorm at 6 years, Journal of the American Medical Informatics Association, Volume 18, Issue 4, July 2011, Pages 441–448, https://doi.org/10.1136/amiajnl-2011-000116
Close - Share Icon Share
Abstract
Objective In the 6 years since the National Library of Medicine began monthly releases of RxNorm, RxNorm has become a central resource for communicating about clinical drugs and supporting interoperation between drug vocabularies.
Materials and methods Built on the idea of a normalized name for a medication at a given level of abstraction, RxNorm provides a set of names and relationships based on 11 different external source vocabularies. The standard model enables decision support to take place for a variety of uses at the appropriate level of abstraction. With the incorporation of National Drug File Reference Terminology (NDF-RT) from the Veterans Administration, even more sophisticated decision support has become possible.
Discussion While related products such as RxTerms, RxNav, MyMedicationList, and MyRxPad have been recognized as helpful for various uses, tasks such as identifying exactly what is and is not on the market remain a challenge.
API
Application Programming Interface - A particular set of rules and specifications that a software program can follow to access and make use of the services and resources provided by another particular software program that implements that API.
CCD
Continuity of Care Document - An XML-based markup standard intended to specify the encoding, structure, and semantics of a patient summary clinical document for exchange. The CCD specification is a constraint on the HL7 Clinical Document Architecture (CDA) standard.
CDA
Clinical Document Architecture - An XML-based markup standard intended to specify the encoding, structure, and semantics of clinical documents for exchange.
Clinical Drug
A name specifying ingredient, strength, and form of a medication.
CUI
Concept Unique Identifier - A meaningless number representing a set of names whose meanings are considered equivalent for a given purpose.
HITSP
Health Information Technology Standards Panel - A combined public private advisory committee on health information technology standards.
HL7
Health Level 7 - A standards development organization influential in health information technology.
IN
Ingredient - The term type (TTY) indicating that this name is that of the substance represented in an RxNorm name responsible for the medicinal activity. Also, the name and the substance.
MIN
Multiple Ingredients - The TTY indicating that this name is that of the ingredients of a combination product represented in an RxNorm name, where those ingredients are responsible for the medicinal activity. Also, the name and the substances.
NDC
National Drug Code - A coding system established by the Food and Drug Administration to track packaged products.
Normalized Name
A name created by a set of formal rules and logic. In RxNorm, the normalized name allows linking of multiple names at a given level of abstraction.
PIN
Precise Ingredient - The TTY indicating that this name is that of the substance, expressed more precisely as a salt or ester of the ingredient, represented in an RxNorm name. Also, the name and the substance expressed precisely.
SBD
Semantic Branded Drug - The TTY indicating that this name is the normalized name created for a branded clinical drug. The name consists of ingredient, strength, and dose form, followed by a brand name in square brackets. Also, the name and the product.
Introduction
RxNorm is a standard nomenclature developed by the United States National Library of Medicine (NLM) in the field of medications. By choosing to represent medications at the level of ‘clinical drug,’ defined as ingredient(s), strength(s), and dose form, it provides normalized names for these clinical drugs, and related drug names, and links its names to other commonly used drug vocabularies. Normalized names are formed by editors using a set of business rules and validations to create names in a standard way, based on the three elements of clinical drugs. The RxNorm system then assigns named distinct relationships linking various concepts (eg, from clinical drugs to the ingredients) algorithmically, and represents associated information such as the Food and Drug Administration's National Drug Codes (NDC) as attributes of the concepts. The most recent RxNorm dataset includes more than 61 000 non-obsolete unique RxNorm drug names (RxNorm January 2011 release). The RxNorm vocabulary is available at no cost from http://www.nlm.nih.gov/research/umls/rxnorm/.
Typical uses of RxNorm include navigating between names and codes among drug vocabularies, exchanging standard RxNorm names and codes, and using information available within RxNorm to assist with medication-related clinical decision support.
The creation of RxNorm was motivated by the need for a single, standard, multipurpose terminology for representing medications. Many clinical information tasks can benefit from the use of a standard terminology for representing drug information, including creation of electronic medical records (EMR), automated decision support, quality assurance, healthcare research, reimbursement, and mandatory reporting.1 The NDCs have not proved suitable for such use. They lack many of the desirable characteristics for controlled terminologies.2,3 For example, the identifiers are not meaningless, but are composed of identifiers for the manufacturer or packager, the product, and the package size. While usable for tracking products, these codes are not suitable for aggregating products for the uses mentioned above. Various drug terminologies, while working well on their own, present a barrier when medical information systems containing these varying names and codes need to be cross-linked or reconciled.4
In 1998, the HL7 Vocabulary Technical Committee created a subcommittee to explore possible sharing of terminologies among pharmacy system knowledge base vendors. They proposed a hierarchical model for representing drug terms that includes a specification for creating formal definitions.5 A key concept in the model was the notion of a clinical drug—a drug as it appears in a provider's medication order, comprised of active ingredient, strength, and dose form.6 Various efforts followed to investigate the validity of such a model. Cimino et al mapped clinical drug terms (53% for overall match) against three leading pharmacy system knowledge base vendors.7 Nelson et al parsed 70% of the entries in the Veterans Administration National Drug File algorithmically into the semantic normal forms (normalized names) of clinical drugs.8 These observations and findings led to the development of RxNorm, a terminology that is intended to represent drug terms in a formalized fashion and support interoperability among various drug terminologies.
RxNorm is built upon what is already available—various drug vocabularies commonly used in pharmacy management and drug interaction software. It is built by creating normalized drug names based on the information and names in the contributing source vocabularies. It has a limited and controlled scope—the domain of medications expressible as clinical drugs. Medical devices or medical supplies are thus not within its scope, whereas non-prescription, over-the-counter medications are included. With the release by the FDA of the labels for over-the-counter products, the number of clinical drugs in RxNorm increased considerably.
RxNorm started in 2002 as an investigational project within the larger Unified Medical Language Systems (UMLS) project. After it was demonstrated that it was feasible both in terms of cost and structure to proceed, the NLM based team (see Appendix below for additional members) developed an independent editing and production database. In November 2004, RxNorm was first released as an independent terminology, and established a monthly release schedule. In October 2008, weekly releases were introduced as additions to the corresponding monthly release. Over the past 6 years, source vocabularies included in RxNorm have grown from 5 to 11, its data size has increased fivefold, and its adoption has grown substantially.
Today, RxNorm continues to evolve as a standard for clinical information exchange. Any source vocabulary included in RxNorm can be used to achieve compliance with the ‘Meaningful Use’ requirements for electronic health records (EHR); such designation establishes an important bridge to full RxNorm adoption.9 Recommended by the Healthcare Information Technology Standards Panel (HITSP), RxNorm is the designated vocabulary to represent ‘Medication Brand Name,’ ‘Medication Clinical Drug Name,’ and ‘Allergy/Adverse Event Product’ (if the product causing the adverse event is a medication).10
Content within RxNorm
No single drug vocabulary provides complete and interoperable drug names, codes, and relevant information. RxNorm takes the multiple drug names, using them to complement each other and reconcile the conflicts among them. By aggregating and organizing content from various source drug vocabularies, RxNorm can derive a more complete and consistent representation of drug names, codes, and relevant information.
Unique identifiers and normalized names
Unique identifiers and normalized names are the primary mechanism to group together semantically equivalent terms and codes from various source vocabularies. Different names and codes can be mapped to each other if they share the same RxNorm Concept Unique Identifier (RxCUI). Table 1 shows an example grouping of terms and codes.
Example grouping of terms and codes
| RxCUI . | Source vocabulary . | String . |
|---|---|---|
| 309304 | RxNorm | Ciprofloxacin 2 mg/ml injectable solution |
| 309304 | SNOMED CT | Ciprofloxacin 100 mg/50 ml intravenous infusion |
| 309304 | MDDB | Ciprofloxacin IV Soln. 0.2% |
| 309304 | MTHSPL | Ciprofloxacin 400 mg in 200 ml intravenous injection |
| RxCUI . | Source vocabulary . | String . |
|---|---|---|
| 309304 | RxNorm | Ciprofloxacin 2 mg/ml injectable solution |
| 309304 | SNOMED CT | Ciprofloxacin 100 mg/50 ml intravenous infusion |
| 309304 | MDDB | Ciprofloxacin IV Soln. 0.2% |
| 309304 | MTHSPL | Ciprofloxacin 400 mg in 200 ml intravenous injection |
Example grouping of terms and codes
| RxCUI . | Source vocabulary . | String . |
|---|---|---|
| 309304 | RxNorm | Ciprofloxacin 2 mg/ml injectable solution |
| 309304 | SNOMED CT | Ciprofloxacin 100 mg/50 ml intravenous infusion |
| 309304 | MDDB | Ciprofloxacin IV Soln. 0.2% |
| 309304 | MTHSPL | Ciprofloxacin 400 mg in 200 ml intravenous injection |
| RxCUI . | Source vocabulary . | String . |
|---|---|---|
| 309304 | RxNorm | Ciprofloxacin 2 mg/ml injectable solution |
| 309304 | SNOMED CT | Ciprofloxacin 100 mg/50 ml intravenous infusion |
| 309304 | MDDB | Ciprofloxacin IV Soln. 0.2% |
| 309304 | MTHSPL | Ciprofloxacin 400 mg in 200 ml intravenous injection |
Multiple levels of description and relationships
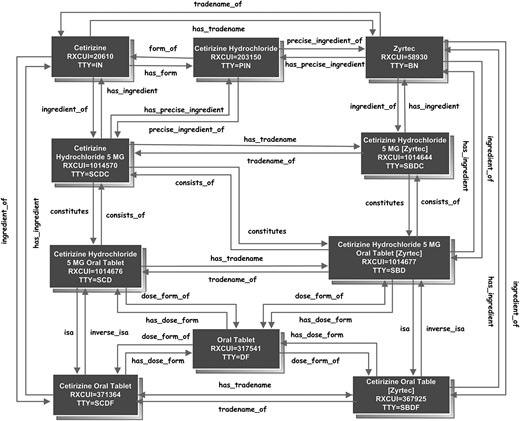
Multiple levels of description and relationships enable RxNorm to represent drugs from multiple purposes or various perspectives. A physician may find the description in the form of a clinical drug useful when he writes medication orders. At the same time, he may find descriptions at the ingredient level helpful for clinical decision support such as drug–drug interaction checking or drug–allergy checking. A pharmacist may find brand names and relationships linking branded names to generic names more helpful when substituting a branded drug with a generic one. A patient sometimes enters a medication for his personal record at the brand name or ingredient level if he doesn't remember the exact strength or dose form. To accommodate these various needs and contexts, RxNorm assigns term types (TTY) to organize various levels of description, representing drugs not only at the clinical drug level (SCD) {ingredient+strength+dose form}, but also at levels of ingredient, which includes single ingredient (IN), multiple ingredients (MIN), and precise ingredient (PIN), clinical drug component (SCDC) {ingredient+strength}, clinical drug dose form (SCDF) {ingredient+dose form}, and pack (multiple clinical drugs or clinical drugs designed to be administered in a specified sequence) as well. Named, reciprocal relationships link a drug among levels of descriptions. These relationships can facilitate clinical decision support or data entry. Table 2 shows the descriptive names of medications across various levels of abstraction. Figure 1 shows the relationships linking an example drug at any various levels of abstraction.
Descriptive names of medications across various levels of abstraction
| Term type . | Descriptive name . | Ingredient . | Strength . | Dose form . |
|---|---|---|---|---|
| IN | Diazepam | ✓ | ||
| SCDC | Diazepam 5 mg | ✓ | ✓ | |
| SCDF | Diazepam oral tablet | ✓ | ✓ | |
| SCD | Diazepam 5 mg oral tablet | ✓ | ✓ | ✓ |
| Term type . | Descriptive name . | Ingredient . | Strength . | Dose form . |
|---|---|---|---|---|
| IN | Diazepam | ✓ | ||
| SCDC | Diazepam 5 mg | ✓ | ✓ | |
| SCDF | Diazepam oral tablet | ✓ | ✓ | |
| SCD | Diazepam 5 mg oral tablet | ✓ | ✓ | ✓ |
Descriptive names of medications across various levels of abstraction
| Term type . | Descriptive name . | Ingredient . | Strength . | Dose form . |
|---|---|---|---|---|
| IN | Diazepam | ✓ | ||
| SCDC | Diazepam 5 mg | ✓ | ✓ | |
| SCDF | Diazepam oral tablet | ✓ | ✓ | |
| SCD | Diazepam 5 mg oral tablet | ✓ | ✓ | ✓ |
| Term type . | Descriptive name . | Ingredient . | Strength . | Dose form . |
|---|---|---|---|---|
| IN | Diazepam | ✓ | ||
| SCDC | Diazepam 5 mg | ✓ | ✓ | |
| SCDF | Diazepam oral tablet | ✓ | ✓ | |
| SCD | Diazepam 5 mg oral tablet | ✓ | ✓ | ✓ |
Relationships linking an example drug at various levels of abstraction.
Attributes
Attributes are relevant information about a drug at an appropriate level of abstraction. This relevant information indicates various aspects of a drug, including those that may be of clinical interest, such as related codes including NDC and Unique Ingredient Identifier (UNII), the new drug application number (NDA), and the abbreviated new drug application number (ANDA). These attributes provide additional information, other than names and codes, about a drug that may be useful for various purposes. While some attributes are listed as those attached to RxNorm names, others are listed as attributes of a given source vocabulary term. This distinction allows the determination if a license is necessary to use that attribute in a system, and allows RxNorm to represent faithfully the data from the source vocabularies it has received.
The National Drug File Reference Terminology (NDF-RT)
The National Drug File Reference Terminology (NDF-RT) was integrated into RxNorm as a source vocabulary beginning with the RxNorm June 2010 monthly release. NDF-RT is a resource developed by the Department of Veterans Affairs (VA) Veterans Health Administration, as an extension of the VA National Drug File.11 The inclusion of NDF-RT has provided RxNorm with an additional type of information. Besides drug names and codes, providers now can find within the RxNorm data the clinical properties associated with certain drugs, such as the possible clinical use, the pharmacologic properties such as the mechanism of action or physiologic effect, chemical structure, contraindications to use, or possible interactions. These clinical properties of a drug may help providers with better decision support. NDF-RT associates clinical properties via named relationships. Table 3 shows the relationships and the represented clinical properties. Table 4 shows the clinical information associated with cetirizine.
Clinical properties and corresponding relationships
| Pharmacologic class | isa |
| Therapeutic intent | may_treat, may_diagnose, may_prevent |
| Contraindications | drug_contraindicated_for |
| Mechanism of action | mechanism_of_action_of |
| Physiology | has_physiologic_effect |
| Metabolism | metabolic_site_of, metabolizes, pharmacokinetics_of |
| Drug-drug interactions | contraindicated_with |
| Pharmacologic class | isa |
| Therapeutic intent | may_treat, may_diagnose, may_prevent |
| Contraindications | drug_contraindicated_for |
| Mechanism of action | mechanism_of_action_of |
| Physiology | has_physiologic_effect |
| Metabolism | metabolic_site_of, metabolizes, pharmacokinetics_of |
| Drug-drug interactions | contraindicated_with |
Clinical properties and corresponding relationships
| Pharmacologic class | isa |
| Therapeutic intent | may_treat, may_diagnose, may_prevent |
| Contraindications | drug_contraindicated_for |
| Mechanism of action | mechanism_of_action_of |
| Physiology | has_physiologic_effect |
| Metabolism | metabolic_site_of, metabolizes, pharmacokinetics_of |
| Drug-drug interactions | contraindicated_with |
| Pharmacologic class | isa |
| Therapeutic intent | may_treat, may_diagnose, may_prevent |
| Contraindications | drug_contraindicated_for |
| Mechanism of action | mechanism_of_action_of |
| Physiology | has_physiologic_effect |
| Metabolism | metabolic_site_of, metabolizes, pharmacokinetics_of |
| Drug-drug interactions | contraindicated_with |
Clinical properties associated with cetirizine
| drug_contraindicated_for | Drug allergy |
| may_treat | Rhinitis, allergic, perennial |
| may_treat | Urticaria |
| has_mechanism_of_action | Histamine H1 antagonists |
| has_physiologic_effect | Decreased histamine activity |
| drug_contraindicated_for | Drug allergy |
| may_treat | Rhinitis, allergic, perennial |
| may_treat | Urticaria |
| has_mechanism_of_action | Histamine H1 antagonists |
| has_physiologic_effect | Decreased histamine activity |
Clinical properties associated with cetirizine
| drug_contraindicated_for | Drug allergy |
| may_treat | Rhinitis, allergic, perennial |
| may_treat | Urticaria |
| has_mechanism_of_action | Histamine H1 antagonists |
| has_physiologic_effect | Decreased histamine activity |
| drug_contraindicated_for | Drug allergy |
| may_treat | Rhinitis, allergic, perennial |
| may_treat | Urticaria |
| has_mechanism_of_action | Histamine H1 antagonists |
| has_physiologic_effect | Decreased histamine activity |
Source content
Source content, if provided to the NLM and indicated as releasable, is present in RxNorm release files. Users wishing to use a source vocabulary integrated with RxNorm may find that, at times, content from a particular source is not available in released RxNorm for a given drug. This can happen for the following reasons:
(a) The source vocabulary does not cover such content at that time. For example, when a drug is approved by the FDA, RxNorm may be the first to create its name and code. Thus, RxNorm would be the only source for this drug when no other source vocabularies have included the new drug in their database and provided that information to RxNorm.
(b) Although the source vocabulary covers the content, such content has not been provided to the NLM. The brand names maintained by Medispan are an example of content that is not provided to the NLM. To obtain these brand names, users would have to consult the source vocabulary provider directly.
(c) The source vocabulary covers the content and provides it to the NLM, but the content is not releasable within RxNorm. Such information includes FirstDataBank's MEDIDs and branded drug names. Additionally, the Center for Medicare and Medicaid Services (CMS) may provide information to the NLM about products not currently covered by other sources, but which need to be represented in RxNorm. As the CMS information is not a true source vocabulary, but simply ad hoc information, it is not released as a source vocabulary. Rather, the normalized names are created de novo by the editors based on the information provided by CMS.
Typical uses of RxNorm
We have observed a steady and fast growth in the use of RxNorm over the past few years, manifested by the increasing number of RxNorm downloads and the vibrant user community activities. Monthly RxNorm downloads averaged about 350 in 2010. There are currently 89 subscribers to the RxNorm listserv begun in January 2010. In addition, we frequently receive inquires, comments, and suggestions from RxNorm users. Typical uses of RxNorm include using RxNorm standard names and codes to capture drug product information in EHR, cross mapping among disparate drug vocabularies, and facilitating medication-related clinical decision support.
RxNorm names and codes
RxCUIs and corresponding names are being included in the CMS Formulary Reference File (FRF) as of the calendar year 2010. RxCUIs are now used in the FRF to represent drug products, replacing proxy NDC codes that were used for the same purpose previously. As each unique RxCUI can represent multiple NDCs of the same drug product, use of RxCUIs can streamline the formulary submission process. Accordingly, Part D sponsors are required to use RxCUIs for formulary submissions.
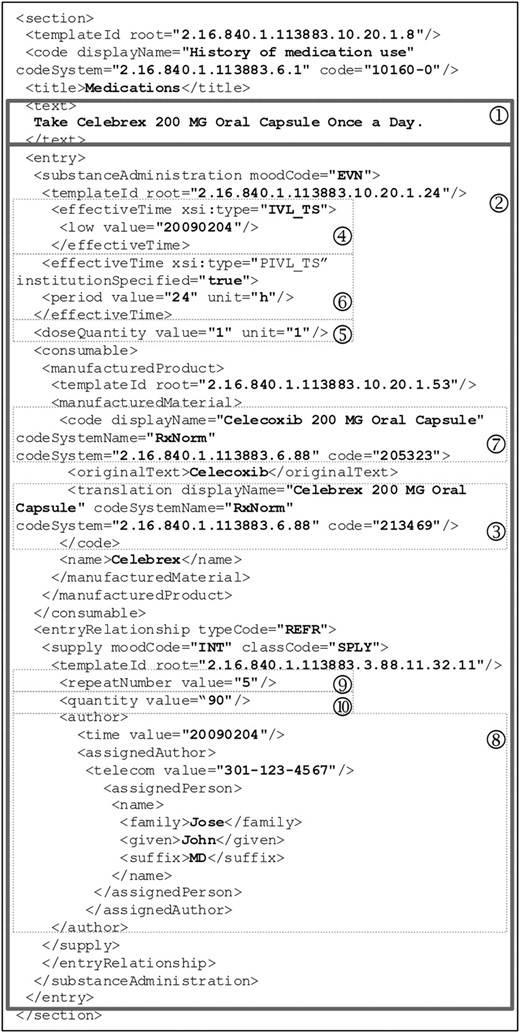
Recommended by HITSP, RxNorm is the designated vocabulary to represent ‘Medication Brand Name,’ ‘Medication Clinical Drug Name,’ and ‘Allergy/Adverse Event Product’ (if the product causing the adverse event is a medication). RxNorm names and codes are used to represent medication names in the Continuity of Care Document (CCD). CCD is a Clinical Document Architecture (CDA) Release 2 implementation that maps the Continuity of Care Record (CCR) elements into a CDA representation, harmonizing CCR and CDA into a common framework.12 CCD is a content standard for patient summary records.9 For easier understanding, an example CCD medication section with RxNorm names and codes and other relevant information is illustrated in detail in figure 2. The medication section contains a narrative block (1) wrapped by the <text> element that renders human readable content and several coded CCD entries (2) for automatic processing purposes. The example document indicates that the patient is currently taking ‘Celebrex 200 MG Oral Capsule,’ identified by RxCUI: 213469 in RxNorm (3). This medication was started on February 4, 2009 (4). Dose is ‘1 capsule’ (5) and frequency is ‘once a day’ (6). The generic counterpart of this drug is ‘Celecoxib 200 MG Oral Capsule’ (7). This medication was prescribed February 4, 2009 by Dr John Jose (8). Number of refills is 5 (9) and quantity dispensed is 90 (10). Note that the branded medication and the corresponding generic drug are represented by RxNorm names and codes.
An example medication section in the Continuity of Care Document body (with prescription history).
Semantic interoperability
RxNorm has been used in the patient data exchange between the VA and the Department of Defense (DoD).13 By mapping from VUID (VA Unique IDentifier) or NCID (Numeric Concept ID used by DoD) to RxCUI, real-time bi-directional encoded data exchange between the two agencies is enabled. Vendors have developed products that support their customers for drug terminology interoperability as well. Some products exploit mappings available in RxNorm as well as those not included in RxNorm. For example, FirstDataBank RxNorm Cross Reference Module provides mapping between RxNorm and NDDF Plus identifiers.14
Medication-related decision support
Standard drug names and codes, and cross-mapping among various drug terminologies can be considered as a necessary first step toward medication-related decision support. That is, correct identification of medications is essential. Based on this, more advanced decision support can be achieved via relationships and attributes in RxNorm. For example, relationships linking the clinical drug and branded drug can be used to discover the unintended duplicate therapy of a branded drug and its generic equivalent. NDCs associated with a drug can be used for dispensing and for inventory checking.
Furthermore, recent inclusion of NDF-RT within RxNorm has made more sophisticated decision support possible. With the drug classification information, medications can be organized into categories and thus what may be duplicate drug therapies within the same class can be identified. Based on the drug indications, a patient problem list can be approximately inferred from his/her medication list. The organization and presentation of patient medication data into drug classes and diseases can facilitate decision support as well as cognitive support.
Users should be aware that while medication related decision support can render many benefits, it can also have significant limitations. For example, drug–drug interaction checking or drug–allergy checking can improve patient safety and lower medication-related cost when combined with computerized provider order entry (CPOE), but it may result in excessive alerting and disrupt clinical workflow.15
NLM-provided RxNorm-related services
To facilitate the use of RxNorm, the NLM has provided a few additional services, mainly developed as research projects, including RxNav, RxTerms, MyMedicationList, and MyRxPad. These services can help users with better understanding of the RxNorm model and content, easier access/retrieval of the RxNorm data, as well as integration of RxNorm into their personal health record (PHR) or EHR systems.
RxNav, the RxNorm Navigator, was originally primarily a browser for RxNorm.16 It is a software application that displays RxNorm names and codes and the relationships among them based on a user's search input. It serves as a supplemental tool to help users browse through RxNorm in a visually friendly and interactive manner. The RxNav Application Programming Interface (API), which was originally developed for RxNav, was made public in 2008. The API serves as an interface to an RxNorm database and provides access/retrieval of the RxNorm data. The API has multiple implementations, including SOAP and REST, making it platform or program language independent. Today, RxNav serves as both a browser and an application programming interface for RxNorm.
RxTerms17 is a drug interface terminology derived from RxNorm to facilitate CPOE. It reorganizes RxNorm names and codes into a two dimensional representation tailored for prescription writing; it eliminates certain drug names that are less likely to be needed in a prescribing environment as well. RxTerms de-normalizes drug descriptions at different levels into an SCD or semantic branded drug (SBD) centric view, associating ingredient, brand name, route, strength, and other related information to the corresponding SCD or SBD. Rather than linking drug descriptions at different levels via named relationships, such association is achieved by organizing a SCD or SBD and its related information into rows of records within a single table of named columns.
MyMedicationList (MML) is an application that helps patients create, update, and save their medication lists.18 MyRxPad is a prototype application that is intended to help prescribers lower some of the e-prescribing adoption barriers and encourage an early positive experience of e-prescribing.19 Both MML and MyRxPad use standard RxNorm names and codes for data entry and recording and save medication or prescription information in the standard CCD format, illustrated in figure 2. They also extract information from RxNorm for medication related decision support, such as auto-completion, over-dose checking at the ingredient level, and linking to prescribing information available at DailyMed (http://dailymed.nlm.nih.gov/). These applications demonstrate the applicability of RxNorm in a PHR or EHR setting. Certain functionalities implemented in MML and MyRxPad can be desirable for other systems, including CPOE with standard RxNorm names and codes, medication or prescription information in the standard CCD format, and some medication-related decision support capabilities.
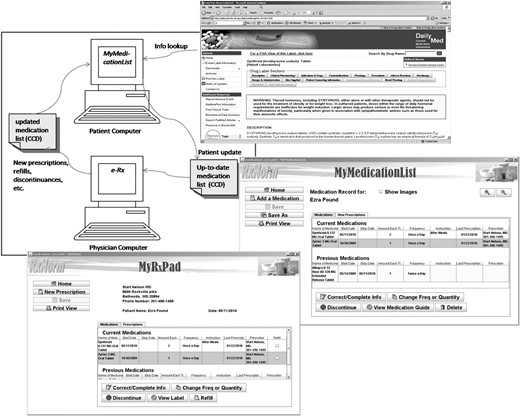
In addition, MML, together with MyRxPad, can serve as an alternative approach to medication reconciliation. A patient uses MML to maintain and update his medication list and shares his medication list (brought in on a USB drive or emailed beforehand) with prescribers. Prescribers use MyRxPad to open the patient medication list, write new or refill prescriptions, and make necessary changes on the patient medication record. Accordingly, the patient obtains an updated medication list that might include refilled medications and other medication adjustments. The patient can then use MML to add the new prescriptions to his ‘current medications' without manually entering the medication names. As the patient carries along the medication list to various prescribers, the evolving and updated list serves as the integrated medication data across disparate providers. As aggregating medication histories from multiple sources may often be difficult,20–22 this patient-centric, participatory approach can address this challenge. MML and MyRxPad provide an alternative approach to medication reconciliation across points of care as illustrated in figure 3. After the initial demonstration phase, more users and organizations are trying out MML and MyRxPad. Researchers at Oregon Health & Science University are using MyRxPad for their research effort to facilitate the access of patient medication records across doctors' offices, long term care facilities, and pharmacies. The WorldVista groups are working to adopt MML and MyRxPad, and use RxNorm for their EMR applications.
MyMedicationList and MyRxPad: an alternative approach for medication reconciliation across points of care. CCD, Continuity of Care Document.
Production and releases
While source content in RxNorm is presented ‘as is,’ RxNorm is not a simple accumulation of the source vocabularies. Source content is integrated into RxNorm through an inversion, insertion, editing, and production life cycle.23 First, content from source vocabularies is converted to a common format which can be processed by the RxNorm system. Second, converted source vocabularies are inserted into RxNorm using various matching algorithms. Third, human editors review all the content that was inserted, creating normalized names where needed. Finally, after the quality assurance process, RxNorm content is released. During this RxNorm data life cycle, semantically equivalent names and codes are grouped, RxNorm unique identifiers, normalized names, relationships, and attributes are generated and assigned, and source content is preserved.
Quality assurance is a major concern in the production of the RxNorm releases. While minimizing requirements for keyboard entry during RxNorm data production keeps typographical errors to a minimum, review of consistency of relationships and other internal checks help assure the quality of the release. One step in the assurance cycle is reviewing where two sources with the same NDC code are linked to different RxNorm names. Reviewing and reconciling these errors provides an important check on consistent creation of RxNorm names and the RxNorm model.
RxNorm is released in full on the working day coinciding with or following the first Monday of each month. Each release follows the rich release format of the UMLS Metathesaurus, and, where references to concepts in the Metathesaurus occur, they refer to the current extant version of the Metathesaurus. Twice a year the releases of the Metathesaurus and RxNorm are scheduled to occur simultaneously, in which case the references in RxNorm are to that release of the Metathesaurus. Weekly releases of RxNorm were begun to keep up with new medications and new formulations when they appear on the market. Most of the information for these additions to RxNorm comes from the Structured Product Labels (SPL) submitted to DailyMed. The weekly release consists of only new material, linked by codes to older material if necessary. Changes in concept structure, such as moving a source atom from one RxNorm concept to another, take place only in the monthly releases, which are full releases.
Distribution, copyright, and customer support
The RxNorm file can be obtained at no cost from the NLM RxNorm website (http://www.nlm.nih.gov/research/umls/rxnorm/). The downloadable zip file contains several RxNorm content files which are bar-delimited text files, as well as load scripts that can be used to import the content files into MySQL or Oracle databases. At the same web site, users can find related information, including the RxNorm overview and technical documentation.
To obtain the RxNorm file, users need to complete a UMLS license agreement, which permits uses of public domain content in perpetuity. Certain content of RxNorm is freely available, including RxNorm names and codes. Other content contains copyrighted proprietary information. For use of that proprietary content, users need to contact the source vocabulary contributor for specific terms of use or licenses.
To be informed about the upcoming RxNorm changes, users can subscribe to the RxNorm announcement at rxnorm-announces-l@list.nih.gov. Inquires, comments, or suggestions can be directed to rxnorminfo@nlm.nih.gov.
Future work
Over the past few years, RxNorm has evolved to be an emerging standard for clinical information exchange. RxNorm names and codes have gained wide recognition. Users often use RxNorm names and codes to record drug names, and RxNorm attributes for various purposes in their PHR or EMR applications. For these specific uses, users may find names and codes from sources other than RxNorm less relevant. Thus, a pre-processed RxNorm subset that contains only data that are pertinent to users' needs seems desirable. The subset can also facilitate the adoption of RxNorm as providers prepare themselves to meet the requirements for ‘Meaningful Use’ Stage 2 when full adoption of RxNorm is expected.
At the time of this paper, the RxNorm team is working on an RxNorm e-prescribing subset. The subset intends to provide a comprehensive nomenclature that includes all prescribable clinical drugs and packs on the market for use in e-prescribing systems. The subset would include Federal Medication Terminologies components (RxNorm, UNII, NDC, NDF-RT classes) as well as important attributes in RxNorm. Not included in the list would be drugs not sold in the US and those sold only for veterinary purposes.
We believe the e-prescribing subset will encourage providers to use standard RxNorm names and codes to enter medication orders electronically. Increasing CPOE use is a core objective specified by the ‘Meaningful Use’ regulation. Only when providers enter orders electronically can the computer help improve decisions by applying clinical logic to those choices in light of all the recorded patient data.24 E-prescribing is a starting point to realize the true potential of EHRs and thus we have named the subset an e-prescribing subset. The use of the subset, however, is not limited to e-prescribing only. The subset also can be applied throughout EHRs when medication information is captured. For example, patients can use the subset to enter medications on their medication lists and pharmacies can use the subset to record the medications dispensed.
Several challenges remain in constructing such a subset. One obstacle is to identify drugs that are on the market. There is no reliable complete and accurate listing of all the drugs that are currently on the market in the USA. We consider the information from the SPLs in DailyMed to be a reliable source for what is on the market. Pending the full listing of SPLs, our approach is to interpret and reconcile source data which provide information on whether a drug is currently on the market. We identify current drugs algorithmically and have editors review the information.
By eliminating names and codes from sources other than RxNorm, the subset size will be much smaller than the RxNorm full release. The smaller file size is desirable for data management and access, as well as for mobile applications.
Like RxNorm, the information included in the RxNorm e-prescribing subset includes not only RxNorm names and codes, but also RxNorm attributes that could assist with medication-related clinical decision. However, both RxNorm and its e-prescribing subset remain as a terminology, not necessarily a complete drug knowledge base. Therefore, certain knowledge is not within its scope, such as formulary information or drug pricing information. Although such knowledge is not included in RxNorm, as more drug knowledge bases are incorporating RxNorm codes, users can choose to interface with certain knowledge bases via these names and codes.
While the basic structure, mission, and uses of RxNorm are well established, additional features and developments continue to be made. Additional drug classification information, allergy and adverse reaction classes, and other grouping information may be included over time.
Conclusion
RxNorm was built with the idea that uses of it might provide substantial benefits including standard names and codes for drug product representation, semantic interoperability across disparate drug vocabularies, and medication-related clinical decision support. At this point it appears that RxNorm is a compliant vocabulary for medications to support ‘Meaningful Use’; incorporation of RxNorm may be needed for health information system certification and for incentive payments.
Acknowledgments
We wish to thank Dr Simon Liu (former Associate Director of the National Library of Medicine (NLM) for the Office of Computer and Communications Systems (OCCS)), Wei Ma (Chief, Applications Branch), and Suresh Srinivasan (Chief, Medical Language Branch) for their contributions on project planning and management. This endeavor is a collaboration between Library Operations (LO) and OCCS within NLM. We wish to thank Sheldon Kotzin (Associate Director of NLM for LO) and Dianne Babski (Head, MEDLARS Management Section) for supporting and coordinating various related efforts. Many other individuals and organizations contributed to the project in various capacities via inputs, comments, or partnership. We wish to thank them and we look forward towards future contributions and collaborations.
Competing interests
None.
Provenance and peer review
Not commissioned; externally peer reviewed.
References
Appendix
RxNorm Editors
George Chang, In Hye Cho, Doris McGinness, Nada Midani, Terry Quinn, Robin Suda
RxNorm Programmers
Shiva Ayyagari, Chandra Kola, Ratnakar Korem, Muse Mekuria, Subbarao Polavarapu, Arunkumar Subramanian
RxNorm Customer Support
Patrick McLaughlin
The work of maintaining the documentation, inverting and inserting multiple sources into RxNorm, as well as distribution of the RxNorm vocabulary and maintenance of the RxNorm website is done by the Office of Computer and Communications Systems (OCCS) of the National Library of Medicine.